Ulenistamab
PBP1510
Conquering Pancreatic Cancer via Novel Therapeutic Antibody

“World’s first targeted antibody therapy for pancreatic cancer”
PBP1510 has received Orphan Drug designation from the FDA, EMA, and MFDS, attaining a comprehensive ODD Grand Slam. Acknowledged as a targeted antibody therapy for pancreatic cancer, it has been awarded the USFDA Fast Track Designation, highlighting its potential as a blockbuster drug.
PBP1510 (INN : Ulenistamab)
Pancreatic cancer commonly exhibits elevated expression levels of Pancreatic Adenocarcinoma Up-regulated Factor (PAUF). Ulenistamab was specifically designed as a targeted antibody against PAUF to impede the metastasis and progression of pancreatic cancer. By focusing on PAUF, Ulenistamab operates within the tumor microenvironment, aiming to restore antitumor immunity that has been suppressed by the metastasis of cancer cells facilitated by PAUF.

Category
Monoclonal antibody
Indication
Pancreatic cancer, Ovarian cancer
Current Status
Phase 1/2a (EU, USA & Asia)
An inaugural human Phase 1/2a clinical trial, conducted at multiple centers with an open-label design, is underway to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of PBP1510 in patients diagnosed with advanced or metastatic pancreatic cancer.
Strength & ROA
PBP1510 400mg/16mL concentrate solution for IV
Approval Pathway
USA
FDA contingent clinical trial approval received in June 2022 and full approval received in November 2022
France
ANSM clinical trial initial approval received in June 2021
Spain
AEMPS initial clinical trial initial approval received in February 2021
Australia
HREC approval received in May 2023
Singapore
HAS approval received in September 2023
Mechanism of Action
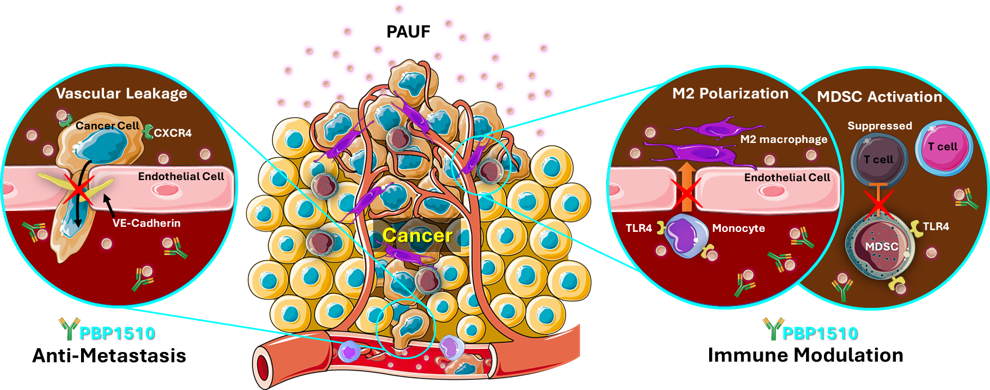
Ulenistamab effectively inhibits the multimodal pathogenic pathway associated with Pancreatic Adenocarcinoma Upregulated Factor (PAUF). PAUF is a tumor microenvironment (TME) modulator which promotes cancer progression and metastasis via multiple pathways by stimulating cancer cell and stroma cell. This humanized IgG1 monoclonal antibody is designed for precise targeting of PAUF, exhibiting high specificity and affinity (Kd 10-9M). By neutralizing PAUF, Ulenistamab disrupts all pathways linked to cancer progression. Notably, when combined with chemotherapy, it demonstrates enhanced treatment potential while maintaining low toxicity.
- Suppression of cancer metastasis: PAUF causes vascular leakage through binding to CXCR4 receptor on cancer cells, allowing cancer cells to escape blood vessels.
- Inhibition of immune evasion: PAUF induces immune evasion through induction of M2 macrophage polarization and activation MDSCs (myeloid-derived suppressor cells)
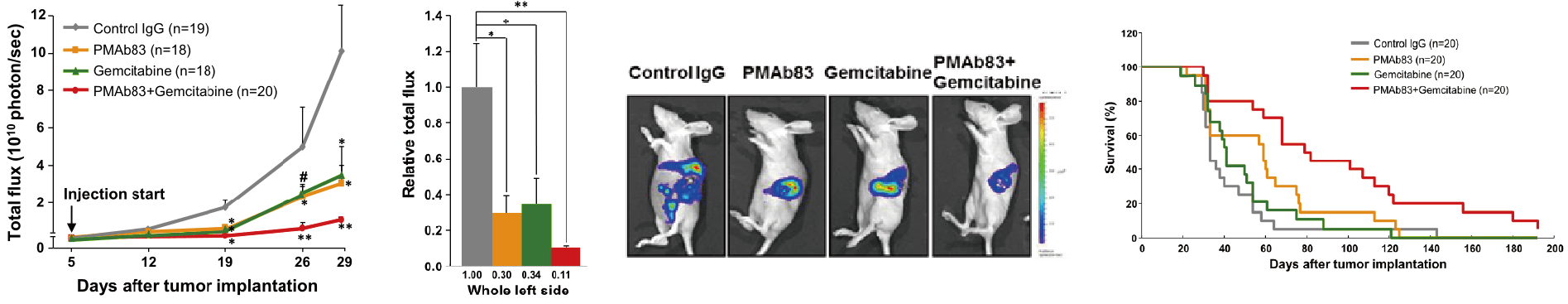
Kim et al, Bio and Biophy Res Comm (2014) 454:144
Ulenistamab, when administered in conjunction with gemcitabine, demonstrated enhanced therapeutic efficacy against pancreatic ductal adenocarcinoma (PDAC) compared to gemcitabine monotherapy. The combined treatment exhibited notable advancements in survival outcomes, a reduction in tumor growth, and a decrease in the incidence of distant metastases.
